

9.2.5 Reproductive and developmental toxicity.9.2.3 Long-term exposure and carcinogenicity.9.1.5 Reproductive and developmental toxicity.9.1.3 Subchronic and chronic toxicity and carcinogenicity.7.3 Cr(VI) levels in the distribution system.7.2.4 Conventional treatment and lime softening.7.2.2.2 Strong base anion exchange resin.7.2.1 Reduction/coagulation/filtration of Cr(VI).6.2.1.2 Field speciation method for Cr(VI).6.2.1 Other methods for determination of Cr(VI).5.6 Multi-route exposure through drinking water.
 4.0 Identity, use and sources in the environment. That leaves glass - even green glass - for beverages continue the package no. It has been detected in none of the cases examined. In our case it was and still is regularly tested, both freshly produced green glass from various glass factories, as well as the bevearages in green glass bottles, specifically to Cr 6+. Several laboratories, including the IGR have already done some test runs regarding this topic. Therefore, only chromium(III) compounds are detectable after the melting process in the finished product. In the glass melt also a raise of the oxidation state from Cr 3+ to Cr 6+ is generally thermodynamically excluded by the commonly prevailing conditions. On top of that, due to the high recycling rate in Germany, also significant quantities of chromium are brought in from the cullets.įirst, the total chromium content is quite low already, second only trivalent chromium compounds such as chrome iron ore are used in central Europe. iron, chromium, however, is actually for some colors difficult to replace. No threshold could be identified for Europe and Germany, thus it can be assumed that here currently a limit of 50 µg/l Cr 6+ applies.Īnd what does that have to do with glass?Ĭhromium is used as a coloring element, mainly in green glass. In our research with regard to Cr 6+ in drinking water, we were able to determine the state of California having a limit of 10 µg/l Cr 6+. This 50 µg/l total chromium are currently used as limit for drinking water in the EU and Germany in the United States generally a limit of 100 µg/l of chromium is used. īut all this means, at least, in the contemplation of chromium, a distinction between these two oxidation states is essential, and only for Cr 6+ can thus a limitation/threshold be useful.įor chromium in total in drinking water, the WHO recommends preliminarily eg. Here, amongst other results, it was gathered clear, that although the human body can convert Cr 6+ to Cr 3+, this process does not work the other way around. The Environmental Protection Agency of the United States ("EPA") has most recently held in September 2013 a workshop with international participants. The Umweltbundesamt examines "actually" (the report is from 2014) the data in Germany on "The importance of hexavalent chromium in drinking water". With regard to the effect in the body (and possble thresholds) there is currently not any valid study that gives a bit of enlightenment. That is why Cr 6+ is also classified such way by REACH and ECHA.Ģ. As long as a risk of cancer is imputed to Cr 6+, it is recommended not to distinguish between oral and inhalation exposure, meaning to take it as being carcinogenic regardless its way in the body. īasically there are two points from previous studies to conclude:ġ. Here, almost all known studies based on a case in China from the 1960s, such as for example was explained in the still recent named study by the Federal Environment Agency of Germany (" Umweltbundesamt").
4.0 Identity, use and sources in the environment. That leaves glass - even green glass - for beverages continue the package no. It has been detected in none of the cases examined. In our case it was and still is regularly tested, both freshly produced green glass from various glass factories, as well as the bevearages in green glass bottles, specifically to Cr 6+. Several laboratories, including the IGR have already done some test runs regarding this topic. Therefore, only chromium(III) compounds are detectable after the melting process in the finished product. In the glass melt also a raise of the oxidation state from Cr 3+ to Cr 6+ is generally thermodynamically excluded by the commonly prevailing conditions. On top of that, due to the high recycling rate in Germany, also significant quantities of chromium are brought in from the cullets.įirst, the total chromium content is quite low already, second only trivalent chromium compounds such as chrome iron ore are used in central Europe. iron, chromium, however, is actually for some colors difficult to replace. No threshold could be identified for Europe and Germany, thus it can be assumed that here currently a limit of 50 µg/l Cr 6+ applies.Īnd what does that have to do with glass?Ĭhromium is used as a coloring element, mainly in green glass. In our research with regard to Cr 6+ in drinking water, we were able to determine the state of California having a limit of 10 µg/l Cr 6+. This 50 µg/l total chromium are currently used as limit for drinking water in the EU and Germany in the United States generally a limit of 100 µg/l of chromium is used. īut all this means, at least, in the contemplation of chromium, a distinction between these two oxidation states is essential, and only for Cr 6+ can thus a limitation/threshold be useful.įor chromium in total in drinking water, the WHO recommends preliminarily eg. Here, amongst other results, it was gathered clear, that although the human body can convert Cr 6+ to Cr 3+, this process does not work the other way around. The Environmental Protection Agency of the United States ("EPA") has most recently held in September 2013 a workshop with international participants. The Umweltbundesamt examines "actually" (the report is from 2014) the data in Germany on "The importance of hexavalent chromium in drinking water". With regard to the effect in the body (and possble thresholds) there is currently not any valid study that gives a bit of enlightenment. That is why Cr 6+ is also classified such way by REACH and ECHA.Ģ. As long as a risk of cancer is imputed to Cr 6+, it is recommended not to distinguish between oral and inhalation exposure, meaning to take it as being carcinogenic regardless its way in the body. īasically there are two points from previous studies to conclude:ġ. Here, almost all known studies based on a case in China from the 1960s, such as for example was explained in the still recent named study by the Federal Environment Agency of Germany (" Umweltbundesamt"). 
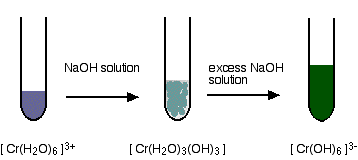
Regarding oral taking, the study situation is far thinner. For the carcinogenicity of inhaled Cr 6+, there are several studies with similar basic statement. The study data on this issue, however, is unbalanced. While the Cr 3+ is not only safe for our bodies, but - at least in certain amounts - even required as a trace element, the Cr 6+ is carcinogenic, which means, that Cr 6+ is supposed to increase the risk of cancer. More than 90% of the chromium in the human body are taken from it by foods such as fish, meat, fruits and vegetables.Īnd why is the topic Chrome then so important? The most important oxidation states of chromium, and content of this document solely, are trivalent chromium (Cr 3+) and hexavalent chromium (Cr 6+). It is found in various forms and various compounds on earth. Chromium in the glass - How, why and which form?Ĭhromium (Cr) is a chemical element from the group of transition elements.







 0 kommentar(er)
0 kommentar(er)
